Photo: Thomas Bastian
Iron deficiency affects an estimated 2 billion people worldwide, including 10–20 percent of young women in the United States. In a pregnant woman, it can also cause lasting damage to the embryonic nervous system.
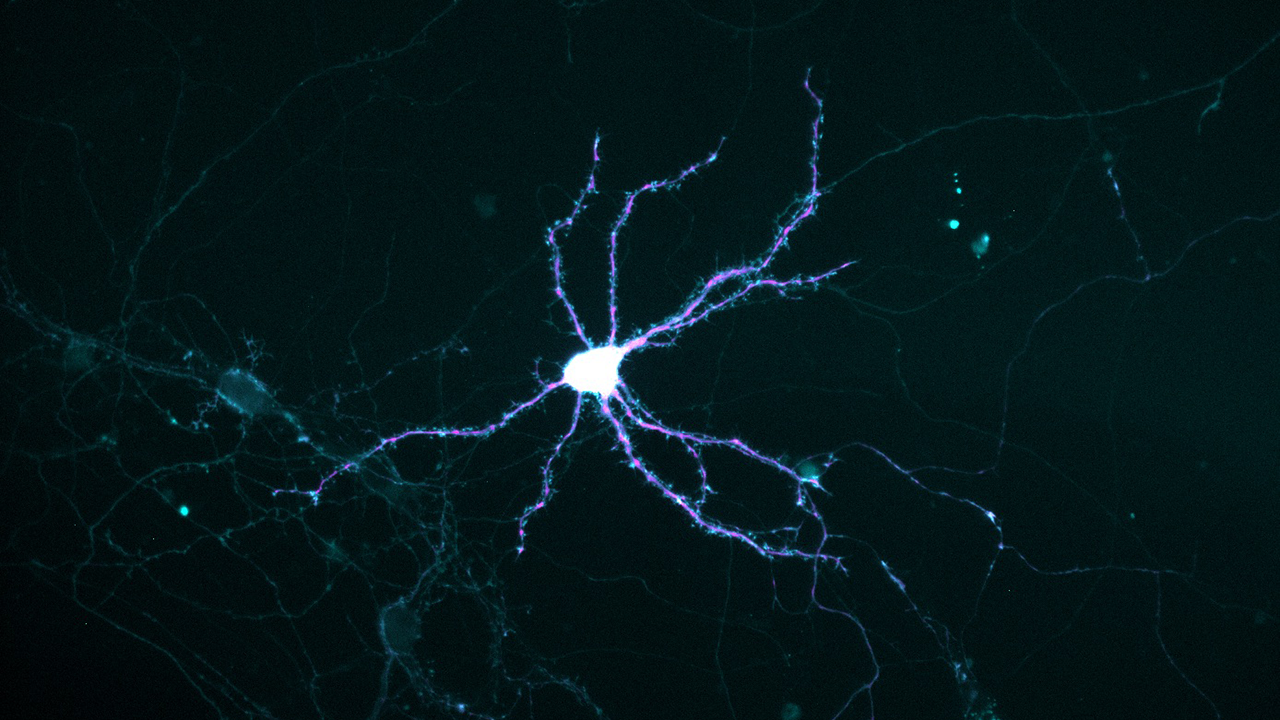
Severe iron deficiency strikes deep in developing neurons, crippling their ability to get enough energy to grow and mature. It counts among its victims neurons of the hippocampus, a deep-brain structure that is central to learning and memory.
“Iron deficiency affects an estimated 40–50 percent of the world’s pregnant women and children and results in long-term neurological impairments, despite iron repletion,” said University of Minnesota researcher Thomas Bastian, Ph.D., a postdoctoral fellow in the departments of Pediatrics and Neuroscience and first author of a new study that reveals how iron deficiency wreaks its silent havoc.
“When it occurs in the womb, it raises the risk of significant psychopathologies, including schizophrenia, autism spectrum disorders, ADHD, bipolar disorder, and depression in the offspring later in life,” he added.
This and other work by Bastian and three U of M colleagues is designed to reveal at what age babies’ brains need iron the most. Also, “We want to find out if there’s some other nutrient—a metabolic stimulant—that can assist iron repletion in restoring normal brain function,” Bastian said.
The study is published in the Journal of Neuroscience.
All About Energy—and a Little History
The researchers focused their studies on the tiny dynamos that generate most of the energy in our bodies. These are the mitochondria, little “organelles” that typically exist by the thousands in a single human cell. They contain enzymes and other proteins that extract the vast bulk of the energy we get from food, and for many of these proteins, iron is an essential component.
Inside mitochondria, the extracted energy is packaged in a small molecule called ATP, the energy currency of our bodies. Just as money makes the economic machinery work by transferring purchasing power, ATP makes our metabolic machinery work by transferring chemical power—for example, to grow, contract muscles, or pass information between nerve cells.
Mitochondria, it is believed, originated as independent, one-celled organisms that took up residence in the cells of our multicellular ancestors as early as a billion years ago. To this day, mitochondria retain a chromosome and can both divide in two and fuse with each other.
Iron Deficiency and Power Outages
During embryonic life, neurons sprout many-branched outgrowths that pick up incoming messages from other neurons. Called dendrites—from the Greek for “tree”—these extensions grow from the central cell body of a young neuron, mature, and connect with other neurons.
To power this activity, a neuron must transport mitochondria to the growing tips of dendrites, like generators being trucked to remote construction sites.
To model the situation in humans near the time of birth, the researchers cultured hippocampal neurons from embryonic mice. They found that in iron-deficient cultures, the stream of mitochondria heading into growing dendrites paused more frequently, resulting in a 42 percent slower speed. Thus, the resupply of ATP failed to meet demand. But no slowdown occurred in the transport of mitochondria in the opposite direction, toward the central cell body, where damaged or unhealthy ones are recycled.
Also, smaller mitochondria became the norm, and their patterns of dividing in half or fusing appeared disrupted. That was worrisome, because a mitochondrion can shed damaged parts by dividing, or gain new parts via fusion.
Future Directions
These findings suggest that mitochondria could be the key to new therapies aimed at protecting young human brains.
Currently, said Bastian, “We’re testing our cultures of developing neurons with various nutrients and drugs that stimulate mitochondria to restore energy metabolism and ATP levels. The big thing is to identify biochemical pathways that drive the neuropathology of iron deficiency so we can target them with nutritional interventions.”