Go to EGMS Home
Sections
1. Sponsor Name
2. Proposal Title
3. Sponsor Due Date
4. RFA/RFP/CFDA/FOA #
5. Submission Medium
6. University Contact
7. Administering Department
8. Principal Investigator
9. Investigator(s)
10. Proposal is for:
11. Proposal is:
12. Proposal Duration & Amount Requested
13. Project Involves
14. SubAwards
15. Conflict of Interest
16. Inventions
17. Space
18. Resources/Spaces/Staff
19. IDC Recovery (ICR sharing)
20. Matching & Cost Sharing
21. Program Income
22. International Component
23. Fairview
24. Proposal Abstract of Executive Summary
25. Notes
26.The PRF's Approval Chain
1. Sponsor Name
A data box is available to collect the sponsor’s name.
A subaward is defined as an award issued under a larger sponsored research award for the procurement of specific services or program-related tasks. Check the SPA webpage at: http://www.ospa.umn.edu/subaward/ for more information. If the University of Minnesota is submitting this proposal to a third party for inclusion in their proposal to a funding agency, click on the “Yes” button. The Sponsor is the third party to which we are submitting our proposal (e.g., the University of Wisconsin). The Prime Funding Source is the funding agency that will fund the project (e.g. NIH).
2. Proposal Title
The Proposal Title reflected on the PRF should be the same as SPA sees on the proposal. The PRF has set aside a generous field to accommodate long proposal titles.
Use the Short Title field to enter an abbreviated title that will help you or rapidly identify the PRF for internal purposes; this field accepts up to 80 characters of text. If the Short Title field is left blank, when the PRF is submitted, it will be titled "untitled request by (creator's x.500)”. The Short Title field appears in several lists in the EGMS forms main menu:
- Status of Requests
- OK/Deny Requests
- Alternate Approver OK/Deny Requests
Adding a descriptive short title helps initiators in locating PRFs on a future date and assists others in finding documents when needed. This field does not appear in EFS nor is it ever sent to a sponsor – it is for internal use only.
Proposal titles reflected on the PRF are subject to the Board of Regents Openness in Research Policy. If the proposal contains proprietary information consider discussing titles with the sponsor to guarantee that there is not a possibility that sensitive information will be disclosed.
3. Sponsor Due Date
The date a proposal is due at the funding institution is added in the Sponsor Due Date field. The Due Time box assists the user and SPA in identifying those proposals with unique time submission constraints and should be added if it applies to the proposal.
Click the radio button that matches the submission requirement when selecting Receipt, Postmark, or Target.
- Receipt is the most common selection and is defined as the date the sponsor requires the proposal to be at the funding institution.
- Postmark is used in those situations when the user needs to show that the proposal was mailed by a certain date.
- Target is used when the sponsor has no defined date; this option is often seen with clinical trial proposals.
4. RFA/RFP/CFDA/FOA#
Critical for SPA, the information collected in this field is used to assist with proposal review. This information is sponsor dependent and not all agencies will have a funding number associated with the submission. It is strongly encouraged to add this data when known but it is not a required field.
5. Submission Medium
There are three distinct submission types for the user to select from: Grants.gov, Electronic, Paper and Paper/Electronic (combination). Each selection has its own unique qualifiers. It is important that the user carefully read the instructions of the sponsor and complete this section correctly as internal deadlines differ between the options.
- Grants.gov-- Grants.gov submissions are designated by selecting this option. Only proposals to be submitted to sponsors using the Grants.gov interface should select this option. For more information about the submission policy for Grants.gov, check the SPA webpage:
http://www.ospa.umn.edu/GrantGov/Howtoapply.html - Electronic Proposals--Electronic proposals are to be entered in this section along with any instructions that are necessary for the Grant Administrator. Some examples of common electronic submissions would include FastLane, Proposal Central or sponsors who only have email as their submission medium.
- Paper only--Traditional SPA requirements still apply for paper proposals. A copy must be included for SPA along with the number of copies along with the original that are to be submitted to the sponsor.
- Electronic/Paper proposals--If the sponsor requires both an electronic and paper copy of the proposal, select this option to enter the information along with any instructions that would assist SPA in processing the PRF.
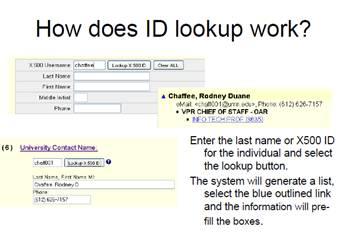
6. University Contact Name
This section has fields for the name, phone number, and email address of the individual knowledgeable about the application that SPA could use as a resource in matters where issues need to be addressed before submission. The system will default to the initiator of the PRF at submission if no other name is entered in the box.
7. Administering Department
This field collects the EFS Dept. ID of the department SPA will use to set up the spending account at award time.
8. Principal Investigator
The Principal Investigator on the PRF should match the individual listed on the proposal being submitted to the sponsor. The individual listed must agree to adhere to the University rules and regulations as set out in the roles and responsibility document found at: http://www.research.umn.edu/regulations/sproles.html.
SPA policy requires that a PI shows 1% effort on the PRF (for all proposal types except equipment proposals) no matter what proposal type. If cost share effort is identified, then it must be further explained with a chart string in section 20 of the PRF at proposal submission time or at time of award. To reduce any delay in award set up, include the dollar amount by the expense category in the comment area on question #20. Effort percentage added should reflect the projected level for the initial budget period.
Approval as principal investigator on a PRF does not imply any commitment on the part of the University to any subsequent appointment beyond what is currently in place. Department and Job titles will flow over to the PRF from the proposal if completed in EGMS Proposal Prep, they can however be modified on the PRF up until the time they are approved. The system provides “lookup” links for both Department and Job Title; it will open a search screen where new information can be found and substituted into the document if needed. The user must first enter some segment of the last name for the “lookup x500 ID” button to access this option.
9. Investigator(s)
Co-PI and Key Personnel are to be designated in the Investigator section. The interface includes checkboxes to indicate whether the individual is Key Personnel and/or Co-PI. If they are Key Personnel but not a Co-PI, an informational email will be generated to inform them that they have been included on the PRF. If the PI box is checked indicating that the individual is a Co-PI they will automatically receive an email asking them to review and approve the PRF. Key Personnel who are not a Co-PI are not required to approve the PRF but can be added to the approval chain if desired.
As with the Principal Investigator, if cost share effort is identified, it must be further explained with a chart string in section 20 at time of submission or at time of award. Likewise, the department and job title can be modified in the PRF if they flowed over from an EGMS Proposal Prep proposal.
Effort percentage added should reflect the projected level for the initial budget period.
10. Proposal is for
This question collects information used primarily for reporting data analyses and to assist SPA in timely processing of the application. All proposal types will fall under one of the headings:
- Research and Development: All research activities, both basic and applied, and all development activities that are supported at universities, colleges, and other non-profit institutions. “Research" is defined as a systemic study directed toward fuller scientific knowledge or understanding of the subject studied. “Development" is the systemic use of knowledge and understanding gained from research directed toward the production of useful materials, devices, systems, or methods, including design and development of prototypes and processes. The term research also includes activities involving the training of individuals in research techniques where such activities use the same facilities as other research and development activities and where such activities are not included in the instruction function.
- Instruction: The teaching and training activities of an institution. Except for research training (outlined above), this term includes all teaching and training activities, whether they are offered for credits toward a degree or certificate or on a non-credit basis, and whether they are offered through regular academic departments or separate divisions, such as a summer school division or an extension division.
- Other Sponsored Activity: Programs and projects financed by Federal and non-Federal agencies and organizations which involve the performance of work other than instruction and organized research. Examples of such programs and projects are health service projects and community service programs.
- Clinical Trials: A research study to test drugs, procedures, or testing technologies to determine whether these are effective and/or safe. SPA needs to know if the clinical trial is either sponsor initiated or PI initiated for the negotiation phase of the project.
- Equipment Only: A proposal for the purchase of equipment only. No other costs are charged against this proposal/award.
- Student Support Only: The proposal is for the costs associated with student support charges only. No other cost is charged against this proposal/award.
- Master Agreement: This proposal is for the costs associated with a master agreement only. A master agreement defines the terms for a single sponsor to fund multiple projects at the University over a period of time.
11. Proposal is:
This field describes the proposal type:
- New: An application not previously proposed, or one that has not received prior funding.
- Revised Proposal (a.k.a. Resubmission): Application previously reviewed. A revised or amended application addresses reviewers’ comments.
- Continuation: An application to award support recommended previously in a funded project. This application is not competitive with new funding requests.
- Renewal (a.k.a. Competing Continuation): An application requiring competitive review by sponsor or peer review committee beyond the current funding period.
- Supplement: Request for additional funds for a current award to expand the scope of work.
- Administrative Change: SPA uses the information to join this proposal to the initial proposal or award or to update existing information when project changes occur. For existing projects, this section collects the agency number and is mandatory if the user has chosen continuation, revised, renewal, or supplement. Additional information on the EFS award number or primary project number must also be added if any of the proposal types listed were a previous award or proposal is currently active.
12. Proposal Duration and Amount Requested
This section is divided into three separate sections dealing with the subject of research funds: duration/amount, award set up, and F&A rate.
- Proposal Duration and Amount Requested:
- The Initial/Current Budget is the initial and/or the current period for which the funds are being requested. This reflects the initial period which may/or may not reflect a year period.
- Entire Budget amount should reflect the current awarded segment of the proposal.
- Department Directions to SPA for Award Set Up:
Users have several options on how a proposal budget will be shared at proposal submission time. The user must select from the three options: Proposal Budget has been established in EGMS, Proposal Budget has been prepared outside of EGMS, orSPA should request a PeopleSoft-friendlybudget from the department at time of award. The selection of one of these three options is mandatory. - F&A Rate:
F&A is defined as Facilities and Administration (a.k.a. indirect costs) and is defined as costs incurred by the University that are not paid directly by the award. For the rate identified, the user must select the base (the budget total, salary and supplies are examples included in a budget total) that is multiplied with the F&A rate to achieve the F&A cost.- MTDC (Modified Total Direct Costs) is also known as the federal rate. This rate charges F&A cost on all direct costs minus equipment, capital (Alterations & Renovations) expenditures, charges for patient care, graduate student fringe, space rental costs of off-site facilities, scholarships and fellowships as well as the portion of each sub-grant or sub-contract in excess of the first $25,000 per project period.
- TDC (Total Direct Costs) is a base which charges F&A on all costs associated with the project
- Other stands for F&A rates that are unique to the sponsor
- More information on F&A rates as they affect the University of MN can be found on the F&A Brochure.
Additional information on F&A is also captured in this section of the PRF. Some sponsors do not allow for F&A costs be added to their proposals, others qualify for a reduced rate; still others are subject to different F&A rates. When an F&A waiver are needed, the user must justify the type, explain the reason, and indicate what type of waiver is being requested. An optional comments box is also available to include further information regarding waivers if needed.
13. Does this project involve any of the following?
When the user has a positive answer to any one of the eight compliance questions, they will be required to review and answer all sections of the question.
Human Subjects
Federal laws and official Regent’s policy require that projects involving the use of human subjects be reviewed and approved by the IRB (Institutional Review Board) to assure compliance with policy that includes both federal regulations and the guidelines of funding agencies relative to the use of human subjects in research. Human subject means a living individual about whom an investigator (whether professional or student) conducting research obtains:
- Data through intervention or interaction with the individual, or
- Identifiable private information
View the Regent’s policy on Research Involving Human Subjects.
For more information on the IRB, see their webpage.
If using controlled substances for research with human, animals, or teaching, check DEHS webpage at: https://dehs.umn.edu/controlled-substances-research
EGMS Proposal Preparation, the PRF, and the MTARF have been updated to accept the entry of the new Human Subjects ETHOS Study ID from the Click IRB system. The id is 13 characters long and begins with the word “STUDY”. The ID you enter will look like this example - STUDY12345678. You can continue to use the older Human Subjects Study Number ID format (10 characters). That number looks like this example - 1702M12345.
Animal Subjects
Federal laws and the official Regent’s policy require that projects involving the use of vertebrate animals be reviewed and approved by the Institutional Animal Care and Use Committee to assure compliance with the Regent’s policy on animal care, federal laws on animal welfare, and the guidelines of funding agencies.
The Regent’s policy on Research Involving Animal Subjects can be found at: https://regents.umn.edu/sites/regents.umn.edu/files/policies/Animal_Care.pdf
Purchase/Use of Custom Antibodies
There is University policy in place to monitor antibodies that have been produced in animals outside of the University. The form to use is available at: http://www.research.umn.edu/iacuc/download/
Human Blood, Body Fluids, or Potentially Infectious Materials
Official University policy along with Federal laws require that research projects involving the use of Human Blood, Body Fluids, or Potentially Infectious Materials are monitored. All researchers working with human blood or body fluids or other pathogens must follow the University’s Exposure Control Plan, and complete the Bloodborne Pathogen Training, available on the web (annual refresher required). Those researchers working with infectious material must follow requirements of the University’s Biosafety Program detailed in the Biosafety Manual and on the Institutional Biosafety Committee’s website.
More information can be found at:
https://ohs.umn.edu/programs
http://www.research.umn.edu/ibc/Stem Cell
University of Minnesota policy states that the Stem Cell Research Oversight (SCRO) Panel must review and approve the following research before it can begin:
- Research with human embryos except for the use of embryos in non-experimental clinical care provided to patients undergoing reproductive treatments.
- Research with human iPS or hPS cells for purposes of making or contributing to an embryo, germ cell (oocytes or sperm), or mixed human-animal entity.
- Research with mixed human-animal entities containing cells derived from human embryos or SCNT, or containing human iPS cells.
The SCRO Panel does not review:
- use of non-pluripotent human stem cells in research
- use of pluripotent human stem cells or human induced pluripotent stem cells other than contributing to an embryo, germ cell, or mixed human-animal entity.
The University policy that covers Stem Cell research can be found at:
https://policy.umn.edu/research/embryonicstemcellsPlease contact the SCRO administration for further information at scro@umn.edu
Recombinant or synthetic nucleic acid molecules, Infectious Agents or Biologically-derived Toxins
The Institutional Biosafety Committee (IBC) reviews University research and teaching activities involving recombinant and synthetic nucleic acid molecules (r/sNA) or other potentially hazardous biological agents. Regardless of funding source, the IBC must review and approve activities prior to initiation of the work. r/sNA activities include exempt and non-exempt work described in the NIH Guidelines, which includes but is not limited to r/sNA transfer into organisms (including human gene transfer), generation and/or use of infectious agents as vectors for r/sNA transfer, and generation and/or use of engineered agents/organisms. Protocols involving human gene transfer require review and approval by the IBC and the Institutional Review Board (IRB).
Other biological agents subject to IBC review include infectious agents (human, animal, plant, etc.) and biologically-derived toxins. Biologically-derived toxins subject to the IBC include those having high acute toxicity (i.e., a mammalian LD50 of less than or equivalent to 100 micrograms/kg body weight) or significant potential for serious subacute or chronic toxicity (e.g., carcinogenicity).Please contact the IBC administration for further information at ibc@umn.edu
For more information:
http://www.research.umn.edu/ibc/
https://regents.umn.edu/sites/regents.umn.edu/files/policies/RecombinantDNA.pdf
Radioactive Materials and/or Ionizing or Nonionizing Radiation Producing Equipment
To be in compliance with the University’s policy on radioactive materials and other ionizing or non-ionizing radiation producing materials, users must identify on the PRF if this is applicable to their proposal. View the Regent's Policy: Radiation Safety.
Chemicals
The use of chemicals is regulated by the University in compliance with state and federal law. Information on the policy along with University guidelines to be considered at proposal submission time can be found at: https://dehs.umn.edu/environmental-health-safety-dehs/lab-research-safety
- For research involving fetal tissue/cells, please review OBAO's information on Fetal Tissue Research oversight and the related administrative policy for research involving fetal tissue.
14. a) Does the proposal include any outgoing subawards? b) Does this proposal include any OTHER planned activity with the community or other outside entities (excluding subawards)?
If the proposal includes any subawards they must be noted. For more information on subawards check the SPA webpage at: http://www.ospa.umn.edu/subaward. Subaward Financial Conflict of Interest information and instructions are located on the SPA webpage at the PHS Subrecipient Financial Conflict of Interest Resource Repository.
For New/Renewal PHS Proposals check to see if your proposed subrecipient is listed on the FDP Clearinghouse of PHS FCOI Compliant Institutions. This list may be found at: http://sites.nationalacademies.org/PGA/fdp/PGA_070596. If your subrecipient is on this list you need only add an annotation following the name of the subrecipient you added on the PRF indicating that they are on the FDP FCOI Compliant list, for example University of Wisconsin (FDP FCOI Compliant List).
If your subrecipient is not on the FDP Clearinghouse of the PHS FCOI Compliant Institutions, follow the instructions on the SPA webpage at PHS Subrecipient Financial Conflict of Interest Resource Repository and add an annotation following the name of the subrecipient you added on the PRF (Question 14) indicating that you have attached Form 1 / Form 2, for example: Subrecipient Name (Form 1/ Form 2 if applicable)
If yes is answered to 14b, the user must complete a follow up question regarding the type of entity/entities will be involved from the list provided. The primary role of the involved entity/entitles must be described in the data box provided for the PRF to pass audit. Queries regarding this question can be directed to the Office for Public Engagement at avp-ope@umn.edu or (612) 624-1706.
15. Potential Financial and Business Conflict of Interest
a) Do you, or your co-investigators, or key personnel (i.e., anyone responsible for the design, conduct or reporting on this project), or a family member (yours or theirs) have a significant financial interest, OR business interest in a business entity that could benefit from the results of this project? See? For help with definitions.
b) Do you, or your co-investigators, or key personnel have a familial connection OR financial or business interest (of any amount) with any proposed subrecipient or collaborator?
Regent's policy requires that academic employees disclose financial interests relating to research or consulting activities at the time of their application submission for research support. The policy can be located at: https://policy.umn.edu/operations/conflictinterest. Information on current approved REPAs also needs to be added to the PRF with the approval date. This information assists SPA with the PRF review and guarantees that the PI is in compliance with University policy.
Definitions are found in the Board of Regent Policy on Individual Conflicts of Interest. Questions can be directed to the Conflict of Interest Program: (612) 626-4727.
16. Inventions
The University of Minnesota defines an invention as: "A patentable invention is any new and useful process, machine, article of manufacture, or composition of matter, or new and useful improvement thereof."
The University Policy: Reporting Inventions or Software Arising from Research
It is the responsibility of the PI to disclose inventions to Tech Commercialization at the time they are created. When SPA sees "yes" checked to any of the four questions, the SPA GA will make note of the potential for inventions and will negotiate the applicable terms and conditions if an award should result from the proposal submission.
Private Commercial information
Private Commercial information means records provided by a submitter that contain "trade secret information" as defined in Minnesota Statute 13.37, Subd. 1(b):
... a formula, pattern, compilation, program, device, method, technique or process... that is the subject of efforts... that are reasonable under the circumstances to maintain its secrecy, and (3) that derives independent economic value, actual or potential, from not being generally known to... other persons...
We are required by law to make available copies of proposals for awarded grants or contracts to the public on request. Investigators who wish to withhold trade secret information from public review should, where it appears in the application, mark it as private.
17. Is sufficient and suitable space to house this project presently assigned to the Principal Investigator’s or other investigator's department or college?
This question allows for the space issue to be addressed at proposal time both by the initiator who creates the form and by all approvers who by signature agree to such usage of resources. The question further identifies the projected space that will be utilized by room number and building name if the proposal is awarded. The space identified should include both the office and lab space involved.
Space allocated to a project at proposal time may come to play with allocating ICR funds, see: Scenarios for Allocating Indirect Cost Recovery (ICR) Funds for policy in these cases.
18. Does project involve University resources, space or staff from more than one department or college?
When resources are to be utilized from more than one department or college, the PRF must be approved by all department heads and deans involved. This question assists with the transmission of information and assures all parties involved have approved the relationship.
The listing of a department/college name does not add an individual to the approval chain of the PRF; the initiator will need to identify the appropriate department head or dean for each unit and add that individual by x.500 ID to the approval chain.
19. IDC Recovery (ICR sharing)
The ICR sharing policy encourages the interdisciplinary nature of research and applies to new and competitive renewals only (existing arrangements are exempt). ICR sharing is mandatory only if the proposal is over $100,000 total cost per year with indirect costs of $1,000 or above with the involvement of more than one intercollegiate center or college. Involved colleges or their representatives must agree on how the returned indirect costs will be shared if the project is funded or they may waive their right to receive a portion of the ICR.
The UMN Policy for IDC Recovery/Sharing: Sharing Indirect Cost Recovery Among Collaborating Colleges/Intercollegiate Centers/University-wide Centers
ICR can be shared by two methods: contribution or separate budgets. Users will consider the following principles to determine the proportion each unit contributes to the project when:
- Contributions should be estimated using a reasonable approach to determine value.
- Estimations of contributions to the sponsored project should be categorized into the three areas: personnel, facilities, and administrative costs.
Departments also have the option to decide not to share ICR revenue. This decision is not mandated by the University; departments make the decision.
A text box for comments is available to share additional information if needed.
20. Matching & Cost Sharing
The terms "cost sharing," "matching," and "in-kind" refer to that portion of the total project costs not borne by the sponsor. These terms are often used interchangeably but attention should be given to sponsor definitions of those terms. The University uses the terms as follows:
- Cost sharing generally refers to labor items.
- Matching generally refers to non-labor items.
Cash funds are usually required by sponsors for equipment acquisition programs, specialized research centers, or other multi-disciplinary programs. Typically, these funds are requested from a college. In-Kind is interchangeable with "matching" but may refer to costs borne by an external organization, for example when individuals at another organization volunteer their time. The administration policy on matching/cost share: Offering Cost Sharing (including Matching and In-Kind Contributions) on Sponsored Projects
When matching or cost share is identified on the PRF, information regarding the contribution needs to be added either in the data box or identified as chart string.
21. Program Income
Program Income is defined in OMB Federal Regulations, 2 CFR Part 200.80, as gross income earned by the recipient that is directly generated by a supported activity or earned as a result of the award. Program income is generally related to income from fees for services performed, the use or rental of real or personal property acquired under federally-funded projects, the sale of commodities or items fabricated under an award, license fees and royalties on patents and copyrights, and interest on loans made with award funds.
Information on program income policies at the University of Minnesota can be found at: Managing Program Income Earned on Sponsored Projects
22. International Component
Many sponsors require that foreign travel be identified and justified as part of the proposal. Noting this on the PRF assists departments and colleges as well as SPA when the proposal is reviewed to check sponsor and University requirements.
If there is a foreign subcontract in the proposal, information on payment terms, exchange rates, and other sponsor requirements must be considered. For more information on International Subawards, check the SPA webpage at http://www.ospa.umn.edu/subaward/forms.htm. To assist users, review checklists for International Subawards #1 and #2 which cover a variety of issues at proposal development time. Check the Policy Library for the most current policy on International Travel.
23. Fairview
All clinical research projects requesting services from Fairview are required to conform to the Clinical Research Budgeting and Billing policy. PIs or delegated staff must use either TASCS (the Time and Study Collection System) or the new system OnCore (Enterprise OnCore) to delineate the services, drugs, devices, tests, and procedures rendered for clinical research.
Enter the TASCS Request or OnCore Protocol number in the field labeled "OnCore or TASCS #". The field allows for the entry of up to 20 characters. Any additional information may be entered in the NOTES (Section 25).
Additional information on the new Clinical Trial Management System (CTMS or OnCore) is located at: https://www.ctsi.umn.edu/researcher-resources/clinical-trial-management-system
24. Proposal Abstract or Executive Summary
This optional text field allows for the PI/staff to include information on the objectives, activities to be undertaken, expected outcomes, and other pertinent details to be included with the PRF to assist reviewers as they examine the form. Text entered in this field will appear on the PDF of the form.
25. Notes
The notes section is an optional field where form participants can share information as it routes through the approval chain. All notes added to the document will print on the PDF and stay as part of the permanent record of the PRF.
The PRF’s Approval Chain
PRF initiators have two options to have their documents reviewed and approved. A paper copy of the form to collect ink is achieved by clicking the “Do not route electronically” box. This option becomes available once the document has fully been audited.
If the initiator chooses to electronically route their PRF, the approval chain is set by the initiator. Add the x500 if known or use the lookup feature with a portion of the last name or x500 and select the appropriate role from the pull down under the Name Role column.
Principal Investigators will automatically appear in the approval chain as the first approver. As individuals review and approve the document, the PRF will display the signature and date making it easy to track. The system will create room for additional signers by selecting the ‘Add Another Approver’ action key.