The Quality Assurance (QA) program supports continuous quality improvement through evaluation, assessment, and action.
QA program quality improvement activities associated with the Institutional Review Board (IRB) performance are conducted in accordance with key program initiatives and Association for the Accreditation of Human Research Protection Programs (AAHRPP) standard I-5.
Standard I-5: The organization measures and improves, when necessary, compliance with organizational policies and procedures and applicable laws, regulations, codes, and guidance. The organization also measures and improves, when necessary, the quality, effectiveness, and efficiency of the Human Research Protection Program.
On this page (jump to):
The University of Minnesota Human Research Protection Program (HRPP) publishes metrics related to IRB activities. These metrics are an important element of tracking and monitoring IRB performance and implementing quality improvement initiatives.
Many program improvements have been made since 2015, including implementation of the HRPP Toolkit and the Ethical Oversight Submission System (ETHOS), an electronic IRB submission system. The metrics provided below are used to measure and report progress toward our goals of enhancing the HRPP program and improving the IRB experience for researchers.
Active Studies
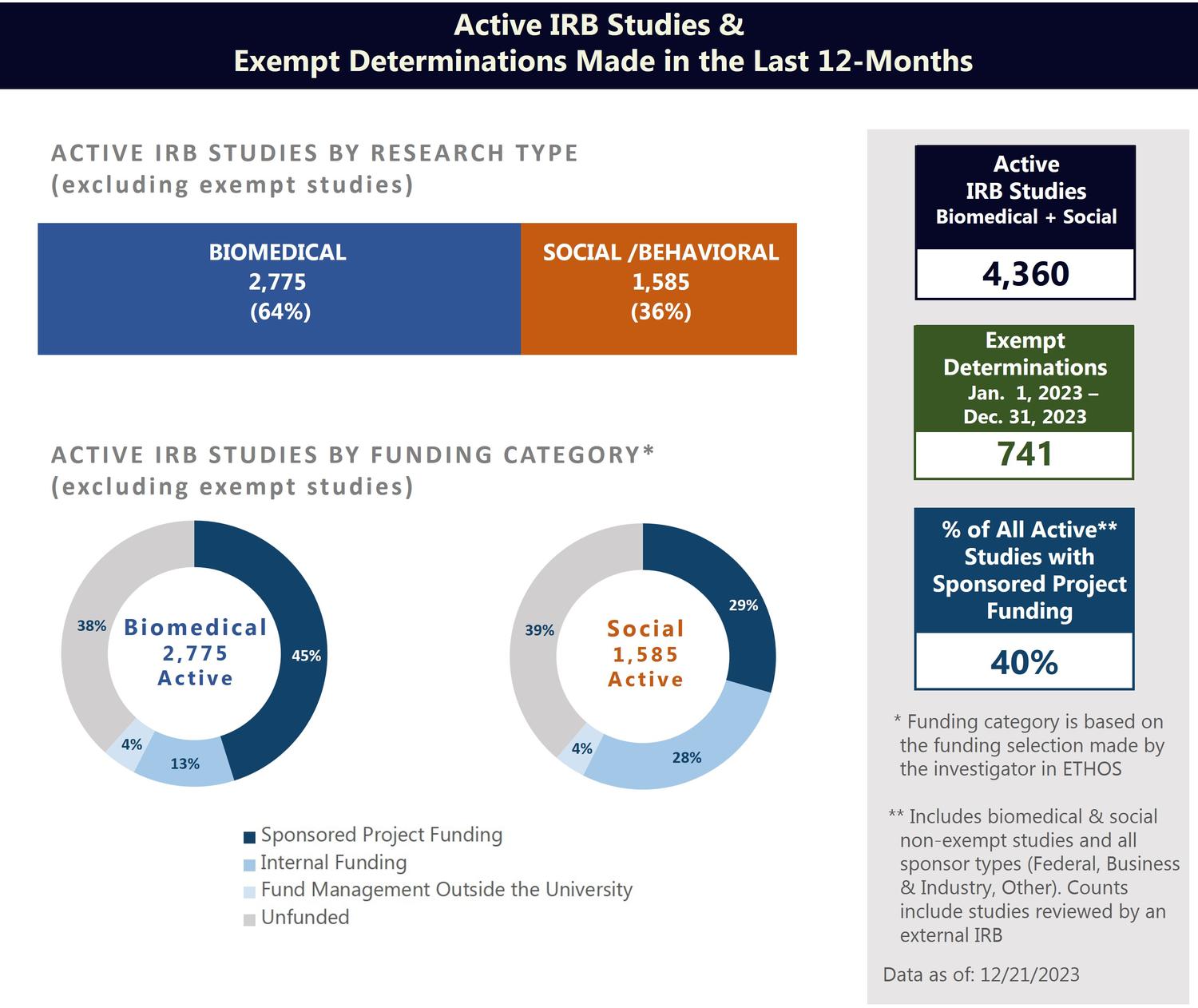
As of December 21, 2023, the IRB had 4,360 active (non-exempt) studies in its records with 40-percent supported by sponsored project funding. In the 12-month period from January 1, 2023 to December 31, 2023, the IRB made 741 exempt determinations.
Monthly Submissions
While the number of active studies in IRB records is a helpful measure, it does not account for the multiple submissions often associated with a single active IRB study.
Total IRB submission volumes is another useful metric, as it accounts for all categories of submissions associated with a study and enables HRPP staff to evaluate and track the IRB’s total workload on a continual basis. IRB submissions can be divided into the following categories:
- New studies (NEW STUDY)
- Modifications to an existing study (MOD)
- Continuing review (CR)
- Minor modifications - add/change personnel (MINOR MOD)
- Reportable new information (RNI)
- Continuing Review with Modification (MOD CR)
- IRB Site for External IRB Studies (IRB SITE)
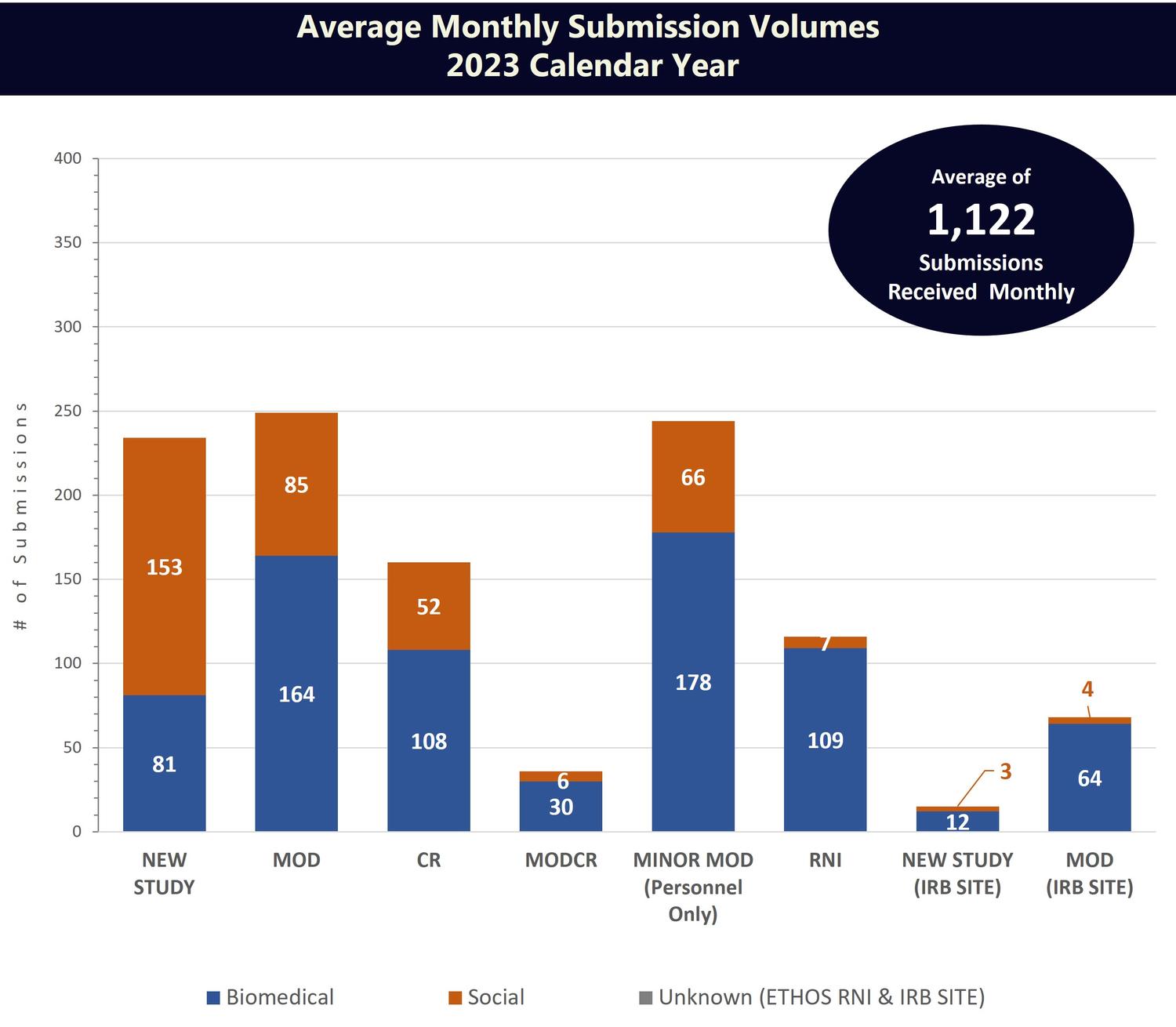
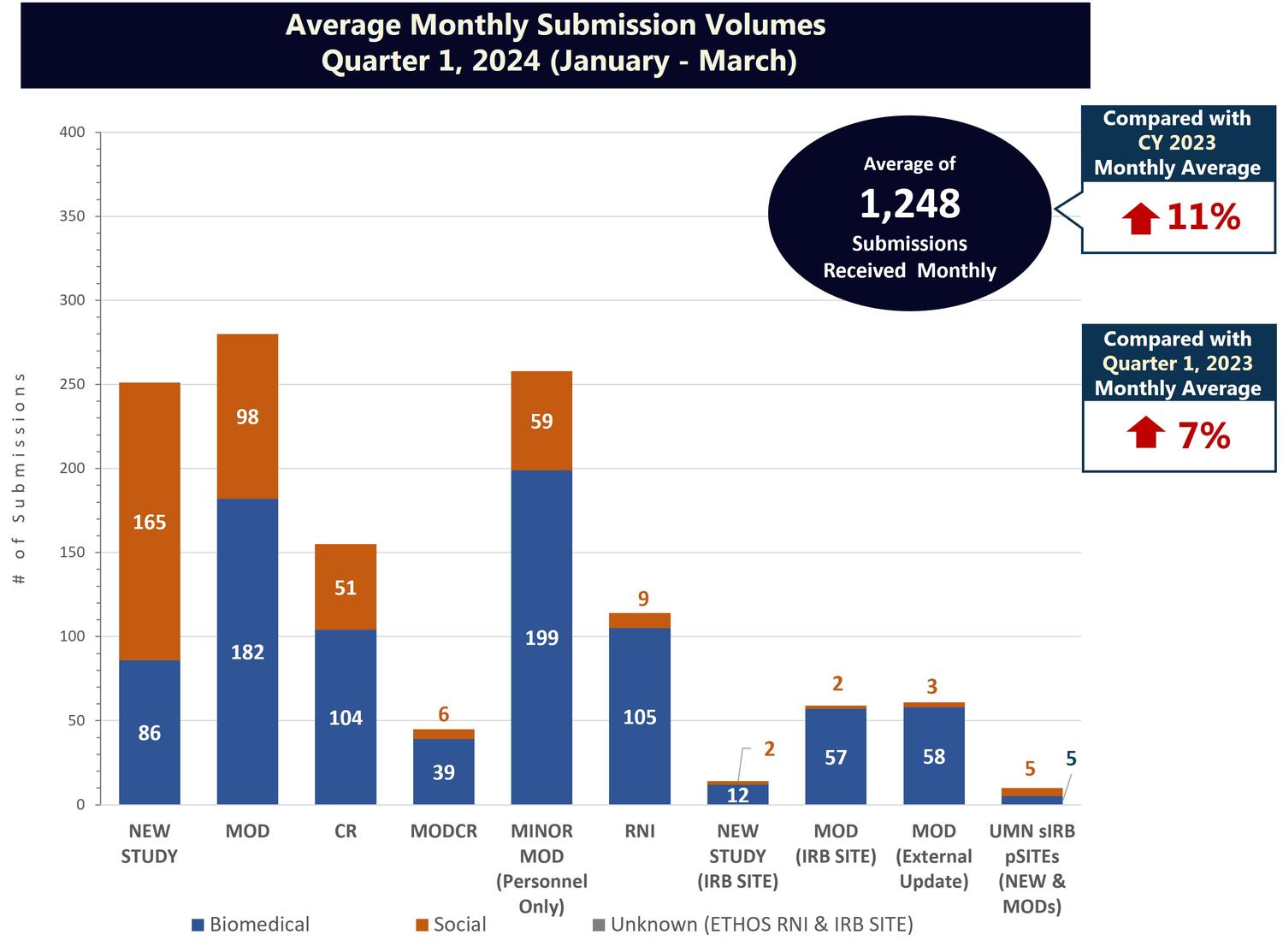
Note that average monthly submission volumes include only the original submission of an item to the IRB. These figures do not include investigator responses to modifications required, responses to deferral, or responses to pre-review clarifications requested. The IRB typically receives an additional 600–700 of these investigator responses each month.
IRB Review
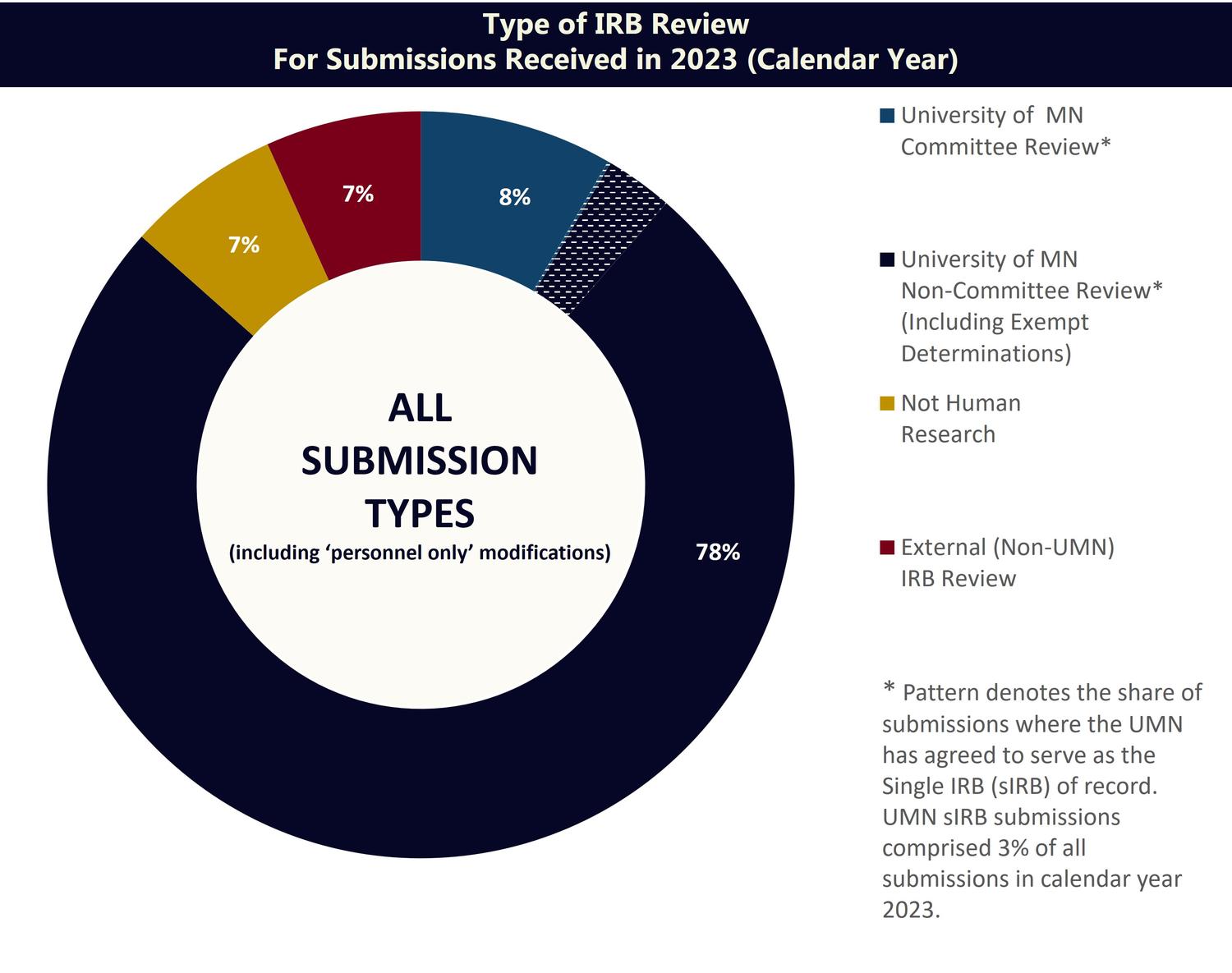
Most submissions to the IRB are reviewed via “Committee Review” or “Non-Committee Review” procedures. A smaller percentage of submissions are reviewed via an external IRB or are determined to be “not human research”. A brief description of each review category is provided below.
Committee Review:
- Full Committee Review: Greater than minimal risk study. Review is conducted by the full board at a convened IRB meeting
- Includes submissions where the University of MN has agreed to serve as the Single IRB (sIRB) of record*
Non-Committee Review:
- Exempt Review: Meets categories of exemption from IRB review. Review is conducted by experienced HRPP staff
- Expedited Review: Not greater than minimal risk study. Review is conducted by at least one IRB committee member
- Includes expedited review submissions where the University of MN has agreed to serve as the Single IRB (sIRB) of record*
External IRB:
- An IRB external to the University of Minnesota conducts the regulatory review and oversight of a study.
Not Human Research Determinations
- The IRB determines activities do not meet the regulatory definition of “Human Research” under any of the following:
- U.S. Department of Health and Human Services (HHS) Regulations
- U.S. Food & Drug Administration (FDA) Regulations
* University of Minnesota Serving as Single IRB (sIRB) of Record: Single IRB review is generally required for federally funded non-exempt human research that is considered multi-site or collaborative. The University of Minnesota may serve as the sIRB of record when the University is the prime grant awardee or may require use of an external IRB (see CHECKLIST: Criteria for UMN IRB Serving as sIRB (HRP-841)).
Turnaround Time
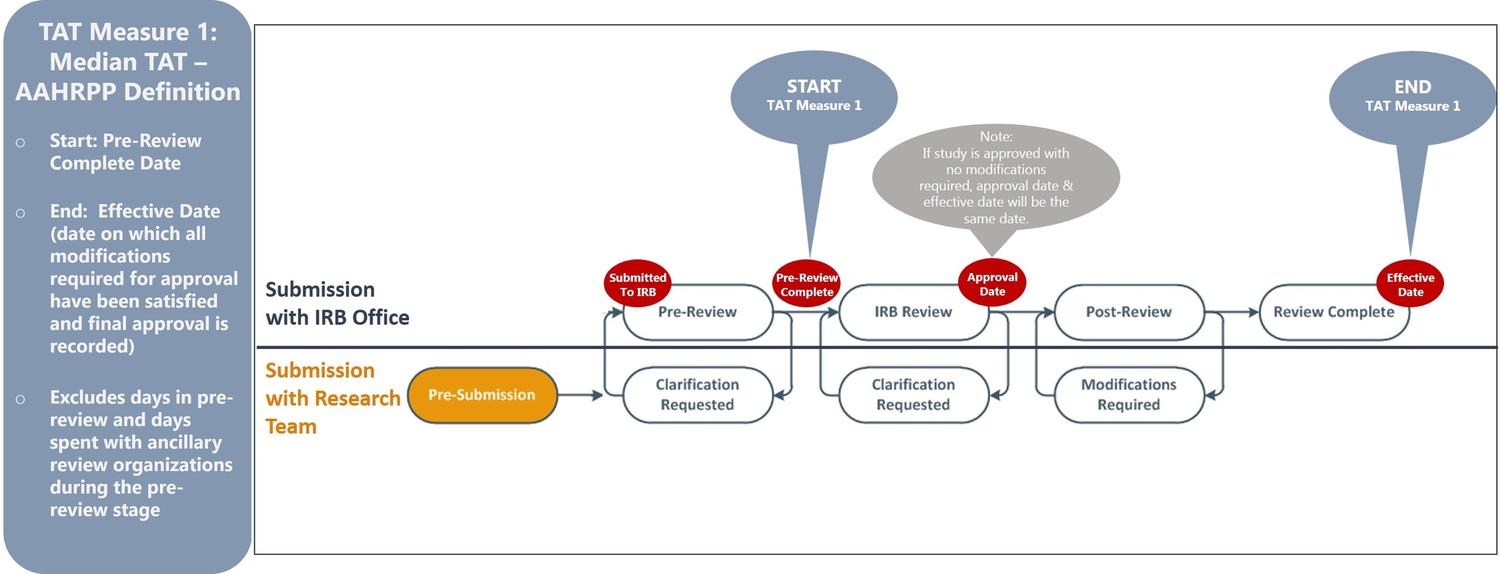
Using data available from ETHOS, the UMN HRPP has elected to publish two turnaround time (TAT) metrics, as described below:
- TAT Measure 1: Enables comparison of the UMN’s IRB performance with other institutions by reporting TATs according to national definitions established by the Association for the Accreditation of Human Research Protection Programs (“AAHRPP”).
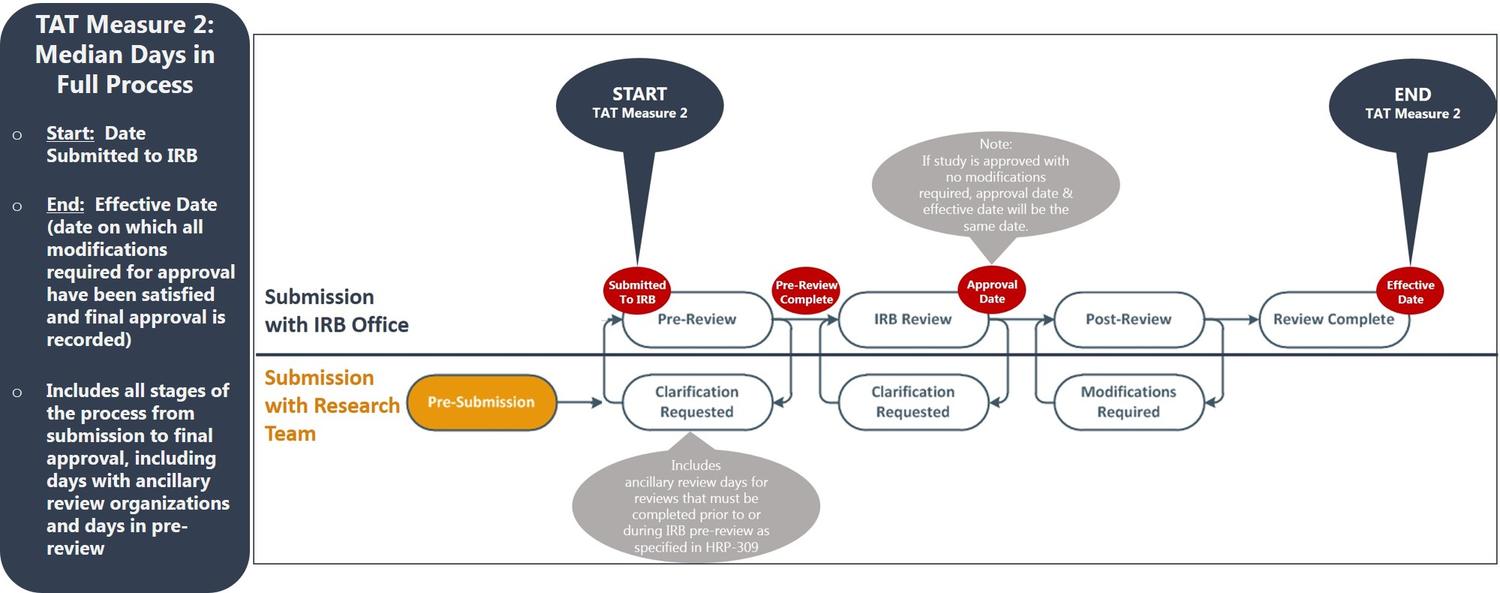
- TAT Measure 2: Aligns with the way in which researchers experience all elements of the University of Minnesota’s local IRB review process. This includes time with other University ancillary review organizations and IRB pre-review.
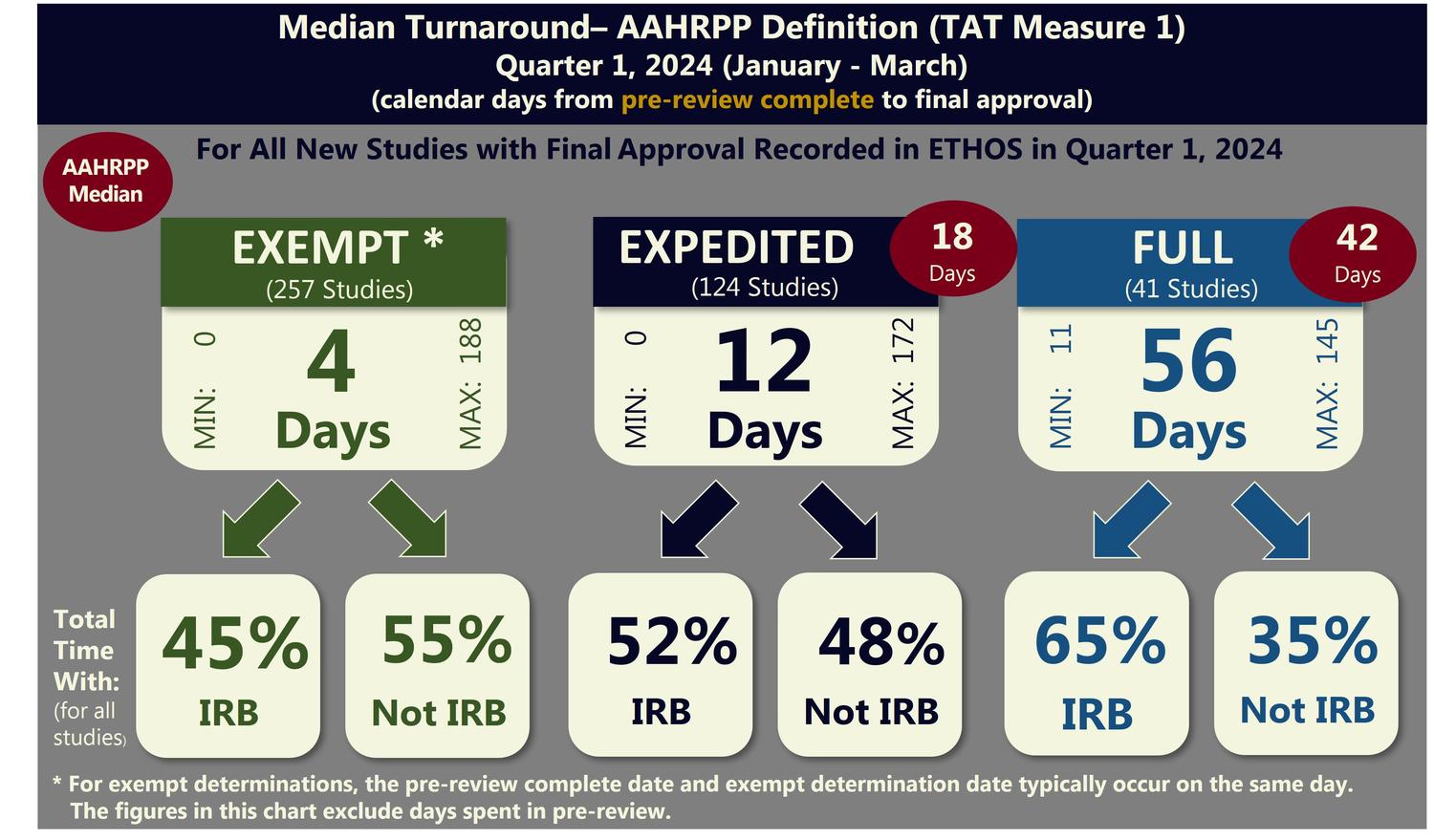
TAT Measure 1 (AAHRPP Definition)
TAT Measure 2 (Full Process)
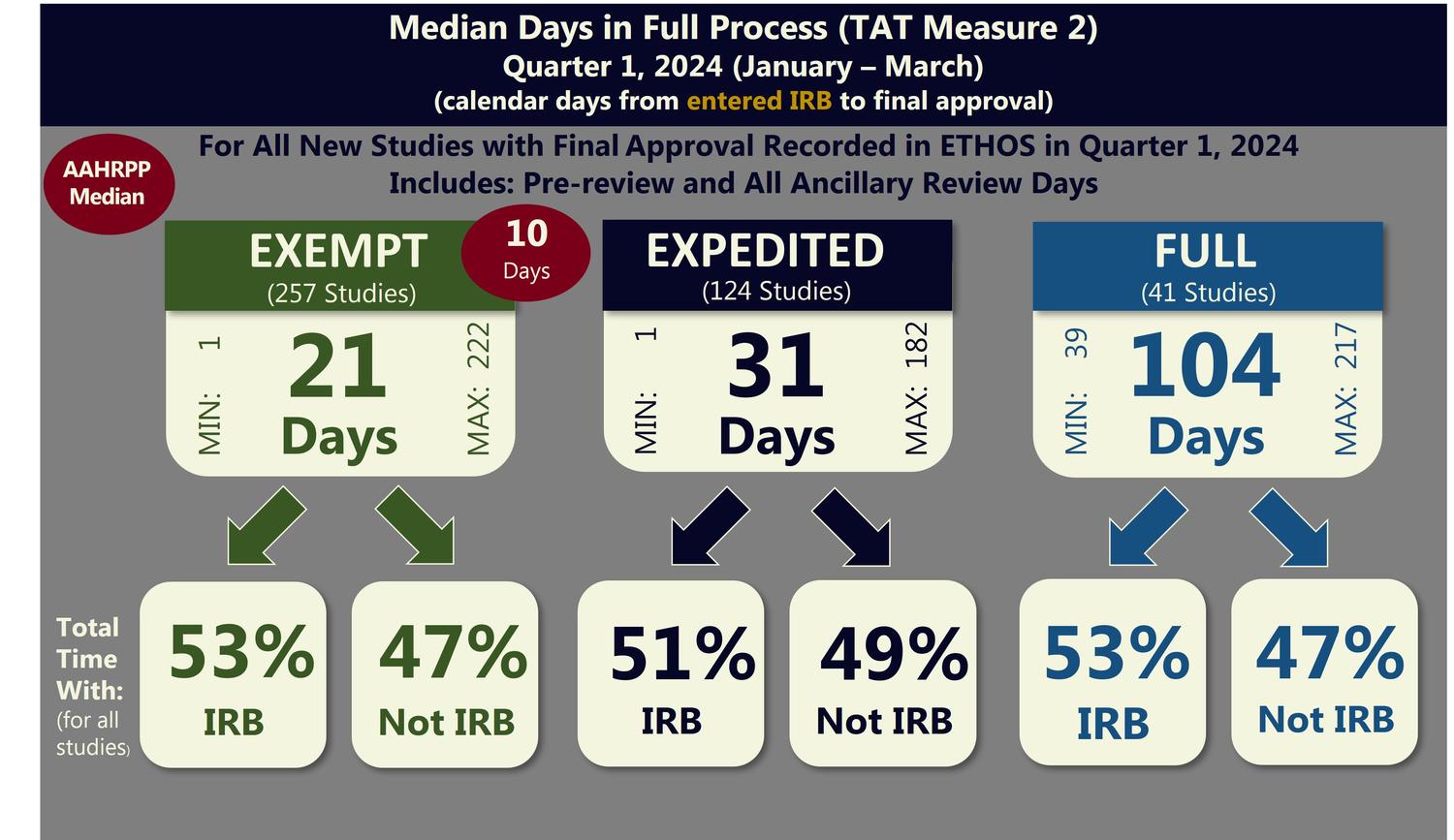
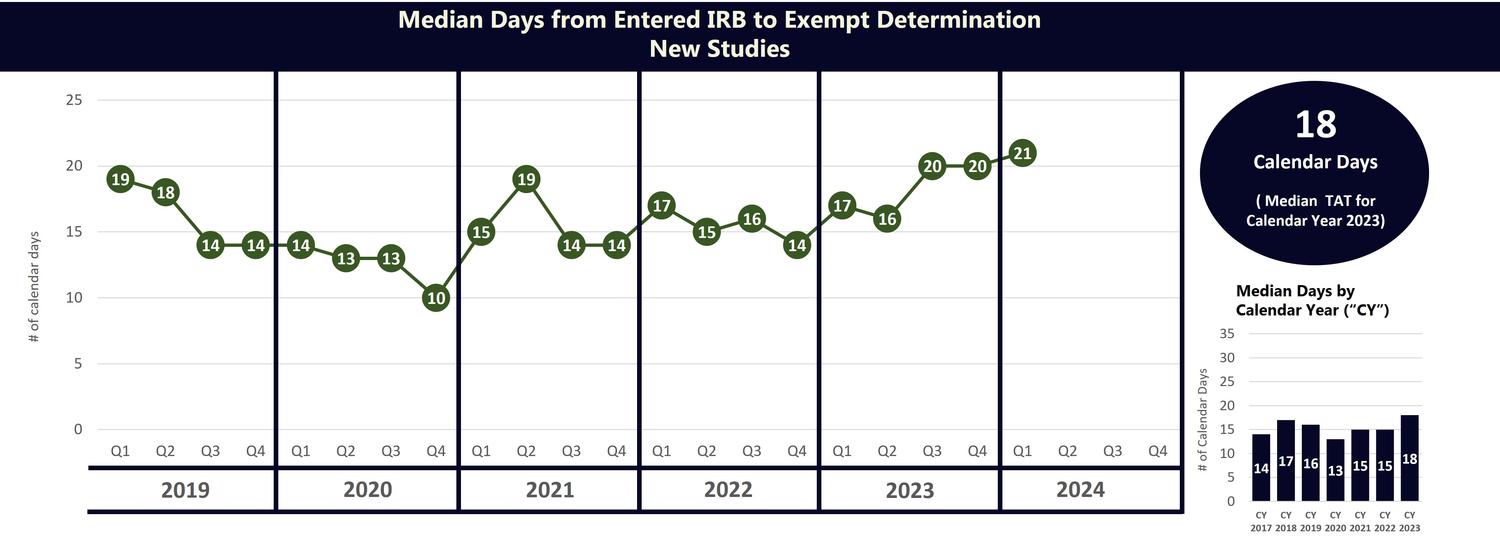
TAT Metrics for Exempt Determinations
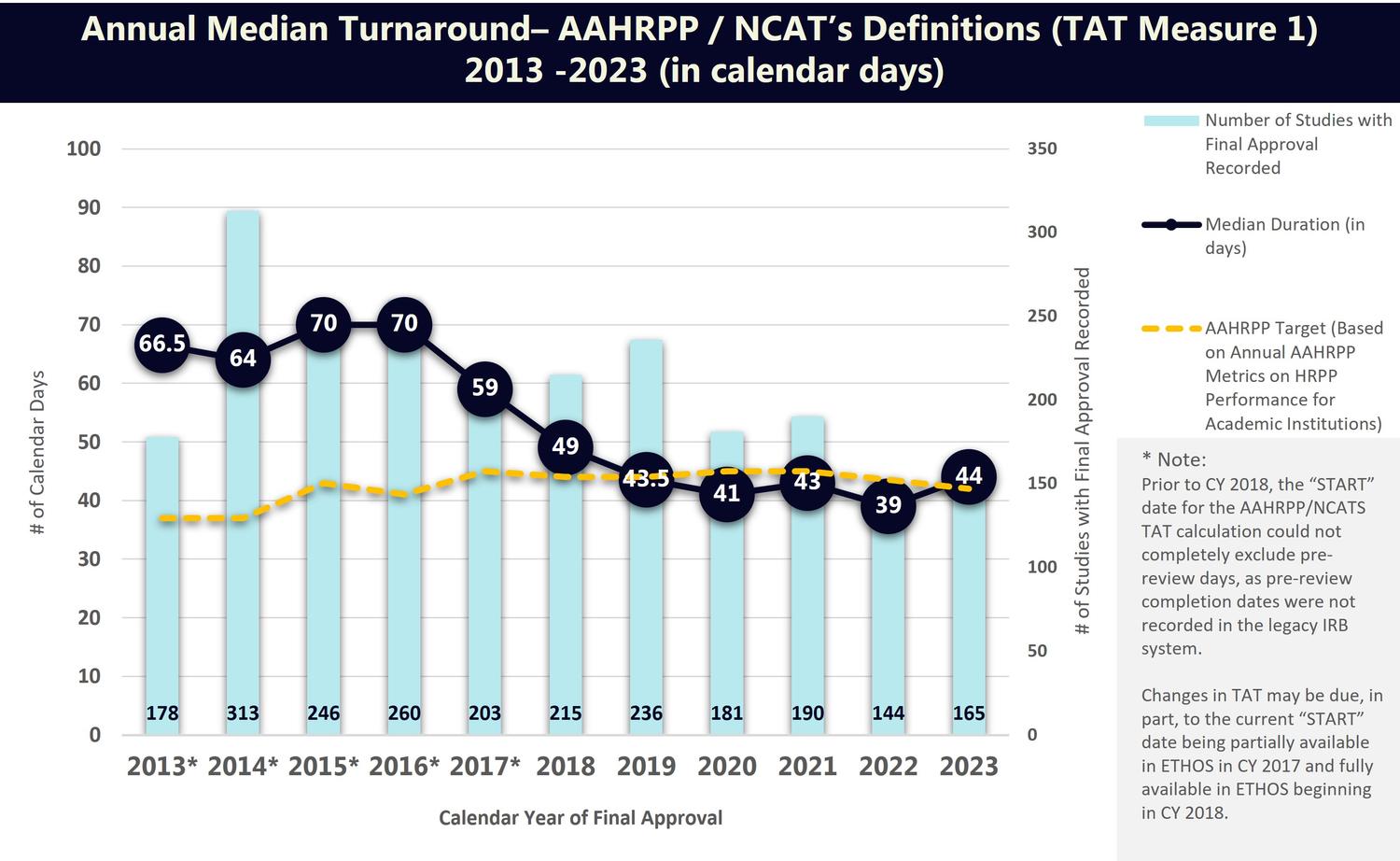
Annual Turnaround Time Metrics
The chart below shows the University’s median turnaround time by calendar year, using the AAHRPP/NCATs turnaround time definition (TAT Measure 1). This chart includes TAT metrics for the period from 2013 through 2023.
Research Community Feedback
The Human Research Protection Program (HRPP) is committed to understanding and responding to the research community’s IRB experiences. As an accredited HRPP through the Association of Accredited Human Research Protection Programs (AAHRPP), it is the HRPP’s responsibility to continuously assess the program and identify opportunities for improvement. The following survey collects research community feedback, and their results inform action plans.
Research Community IRB Experience Survey
This survey is sent to the research community regularly to gather detailed feedback about the research community’s experience with the IRB. Survey questions focus on the quality, efficiency, and effectiveness of the IRB, and results enable IRB leaders to better understand the research community’s perspective and identify strengths and opportunities for improvement. View Previous Survey Results and Action Plans or the most recent survey reports and IRB action plans here*:
- Survey Results - December 2023
- Action Plan and Progress Report - December 2022 Survey
- Comparison of Results Over Time 2019-2023
*A results report was not published for the August 2020 survey due to pandemic-related resource constrictions. However, these results are included in the “Comparison of Results Over Time” report.
IRB Reliance
Single IRB (sIRB) and external IRB are terms used to describe two forms of IRB reliance relationships. These relationships involve formal agreements between institutions, or between an institution and an independent IRB, allowing IRB regulatory review and oversight of a study by one IRB on behalf of another.
More information about IRB reliance is available: Single IRB (sIRB) and External IRB: Reliance Arrangements.
University of Minnesota Serving as sIRB
Most federally funded, non-exempt, multi-site or cooperative research requires the designation of a single IRB (sIRB) to serve as the reviewing IRB for all U.S. based participating sites. As of January 21, 2020, the UMN IRB may serve as the sIRB for federally funded cooperative or multi-site research (see “Checklist: Criteria for UMN Serving as sIRB (HRP-840) (download).”
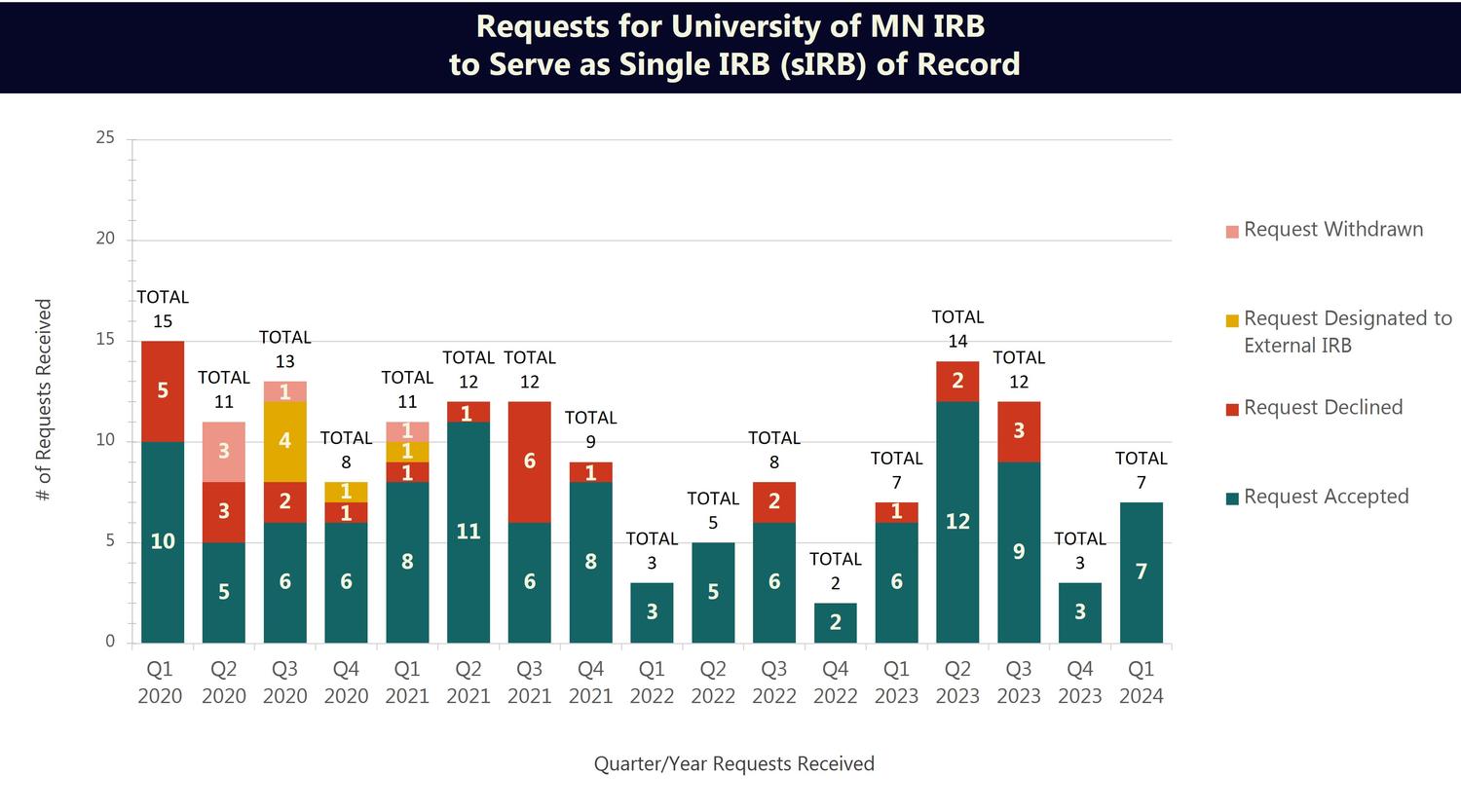
The chart below summarizes the number of requests the University of Minnesota IRB has received, by quarter, to serve as the single IRB of record.
Most recently, in Quarter 1, 2024 (January 2024 – March 2024) the IRB received 7 requests to serve as sIRB. Of those requests, 7 were accepted and 0 were declined.
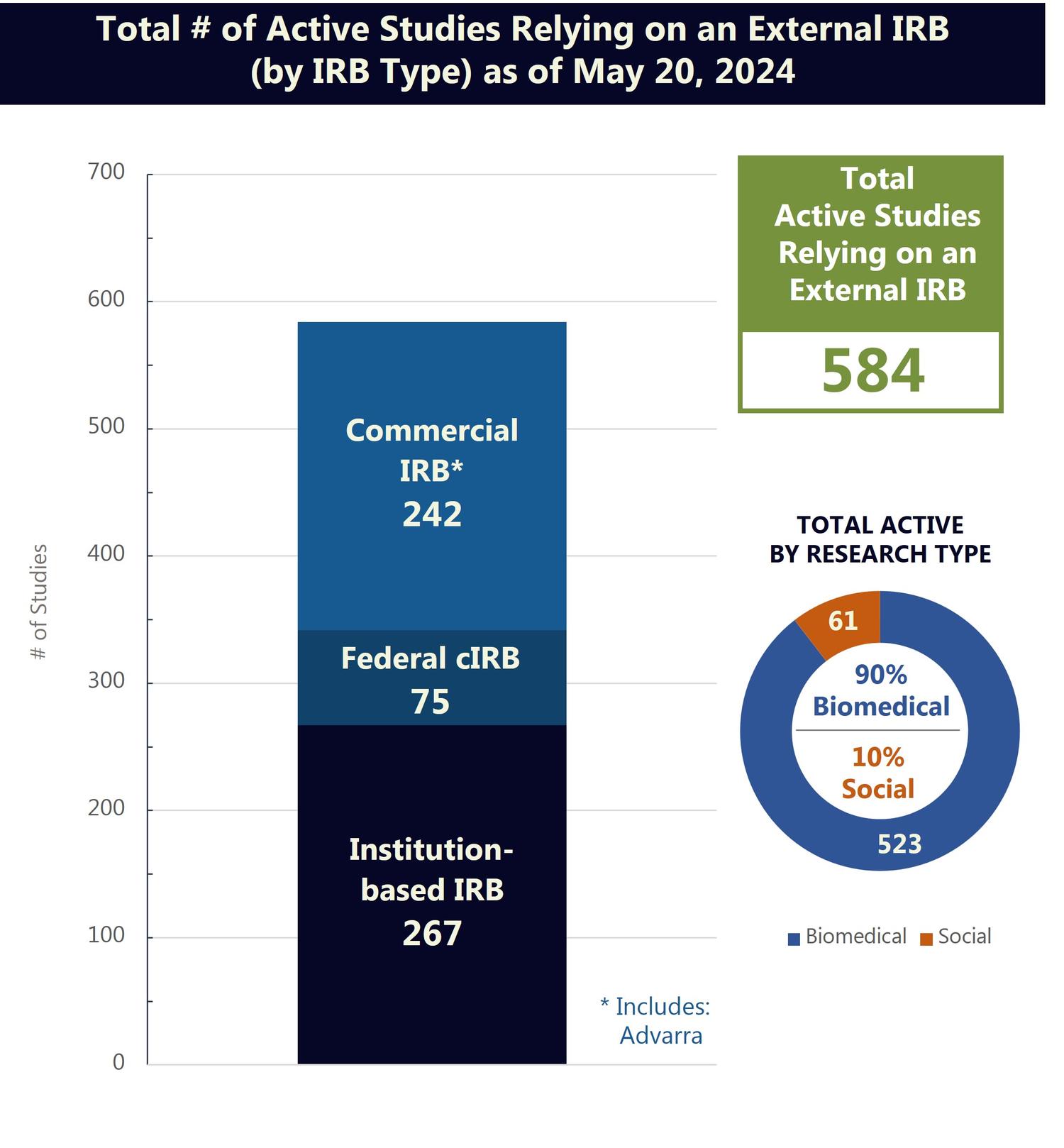
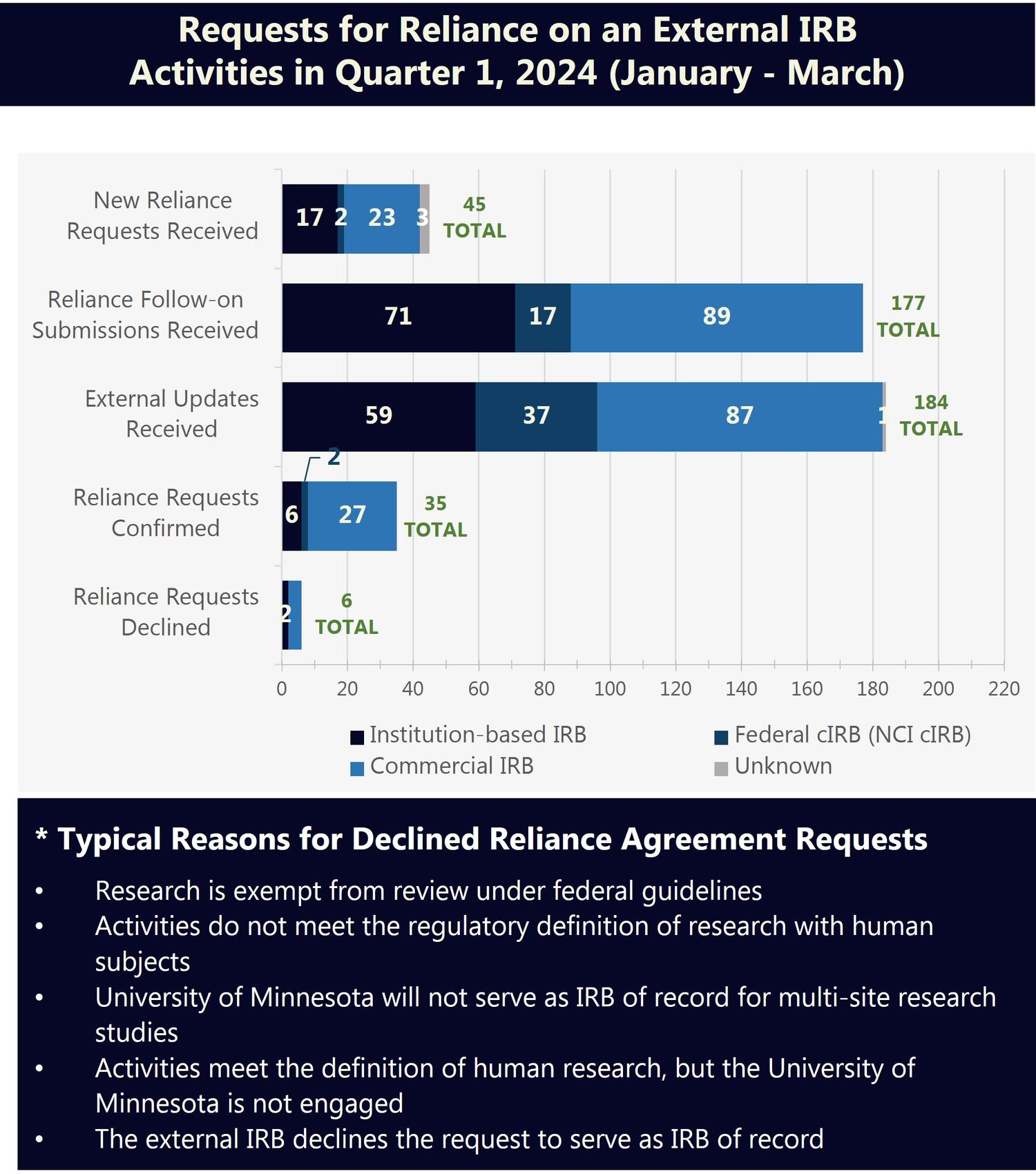
University of Minnesota Relying on an External IRB
Reliance on an external IRB refers to the use of an institutional review board (IRB) outside of the University of Minnesota (external IRB) to conduct the regulatory review and oversight of a study. The University has reliance arrangements in place with several IRBs and is part of a network of over 900 participating institutions that have joined SMART IRB and may use the SMART IRB Agreement to facilitate IRB reliance.
The figures below provide an overview of the current scope of University of Minnesota research relying on an external IRB and recent requests for reliance on an external IRB.