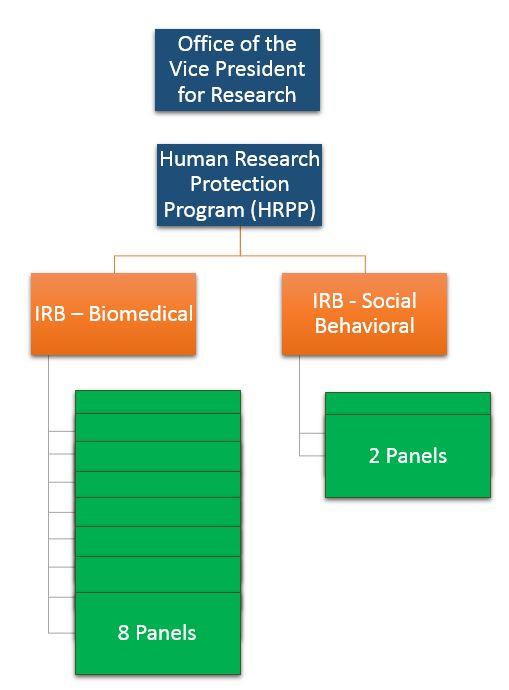
The Institutional Review Board is a key component of the University of Minnesota’s Human Research Protection Program. Other departments that contribute to ensuring the ethical conduct of human research include the Research Compliance Office, Sponsored Projects Administration, the Center for Bioethics and the Health Information and Privacy Office.
University of Minnesota IRB structure
There are eight biomedical IRB panels and two social behavioral IRB panels at the University. The IRBs receive more than 10,000 initial applications, modification requests, reports of new information, or requests for changes to research teams each year. Each IRB biomedical panel meets every other week, while each social and behavioral panel meets at least once per month.
The University IRBs are situated under the Human Research Protection Program (HRPP), a program that reports to the Research and Innovation Office at the University. The Vice President for Research and Innovation also serves as the institutional official (IO).
Each committee consists of faculty, students, and staff representing all University campuses, Fairview, and Gillette Children’s Specialty Hospital. Membership expertise includes scientists (biomedical and social behavioral), nonscientists, and unaffiliated members.
Federalwide Assurance
The University’s IRB holds a Federalwide Assurance (FWA number 00000312) from the Office for Human Research Protection (OHRP) in the Department of Health and Human Services (DHHS). This FWA is a binding agreement between DHHS and the University of Minnesota and is updated and renegotiated at regular intervals.
Often sponsors or agencies request formal documentation regarding the IRB’s FWA and registration. Use this letter for these requests.

Accreditation
The University’s Human Research Protection Program is accredited by the Association for the Accreditation of Human Research Protection Programs. Learn more about AAHRPP accreditation.