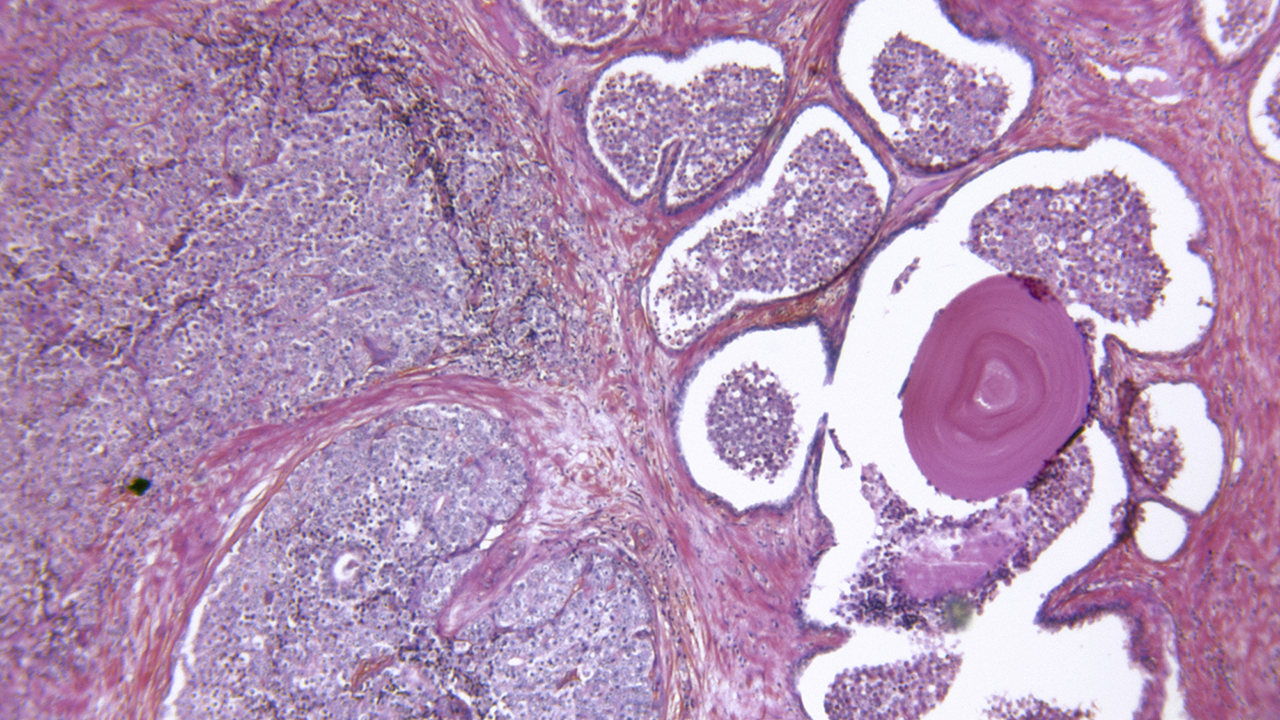
Working with prostate cancer tissue, a University of Minnesota researcher has identified characteristics that separate cancer cells that are invasive—i.e., spreading in tissues of the original organ—from metastatic cells that are gearing up to seed new tumors in distant organs.
The study, by researcher Akhouri Sinha, PhD, paves the way for pathologists and other physicians to more accurately assess a patient’s status and take appropriate measures. It is published in the journal Future Science OA (open access).
“Being able to identify metastatic prostate cancer at the initial point of diagnosis could result in earlier targeted treatment, which could extend the patient’s life,” said Sinha, who recently retired as a professor in the Department of Genetics, Cell Biology, and Development, College of Biological Sciences. “This is the first study to demonstrate differences between invasive and metastatic cell types.”
According to the Centers for Disease Control, prostate cancer is second only to nonmelanoma as the most common cancer among men in the United States. It is a leading cause of death among men of all races. However, Sinha is confident his findings apply to all metastatic cancers.
“It’s an educator’s intuition, based on 50 years of experience,” he said.
Shape Matters
“Metastatic cells are a lineage of stem cells,” Sinha said. Using light- and electron microscopy, he found how these cells differed from their stem cell parents and from invasive cancer cells.
For one thing, metastatic cells have shed their more rounded shapes and become more like 3-D columns or cubes. They also showed deformable, shape-shifting nuclei, a trait often found in stem cells but not invasive cancer cells.
Perhaps most key, Sinha found that the membrane enclosing the cell nucleus had broken down in metastatic cells, allowing genetic material to leak into the rest of the cell. Also, these cells were peppered with dense packets of DNA molecules that now had access to the cell membrane—the outer membrane that encloses all cells.
But, Sinha said, it’s not the metastatic cells themselves that metastasize, or spread, to other organs.
DNA Does the Damage
Cells in the prostate and other organs don’t jostle around like jelly beans in a bag. They are attached to “basement membranes” that hold them in place. In order to metastasize, a cell would have to cut through these membranes, then break into a capillary and out again to establish a metastasis in a new organ.
This is a tall order. Too tall, said Sinha, because all this “breaking and entering” requires large amounts of enzymes called proteases, whose job is to slice up proteins.
“Stem cells alone produce insufficient amounts of proteases to [split open] capillary walls and enter the general circulation and to exit from the capillary to metastatic sites,” he said. “This led us to conclude that the migration of individual invasive cells beyond the prostatic [connective membranes] has many barriers for a successful metastasis.”
He cites the work of researchers elsewhere who found that prostate tumor DNA circulating in the bloodstream carried all the “driver” genetic mutations that caused the original cancer. Since DNA can readily pass into and out of cells—including those that line capillaries—Sinha hypothesizes that the dense DNA packets that show up in electron microscopy of metastatic cells are the true agents that establish metastases in distant organs, provided they carry the requisite mutated genes.
“Once established in cells of liver, lung, pelvic bones, or other metastatic sites, they turn those cells into prostate cancer cells,” Sinha said. “It stands to reason that this is how all cancers metastasize.”