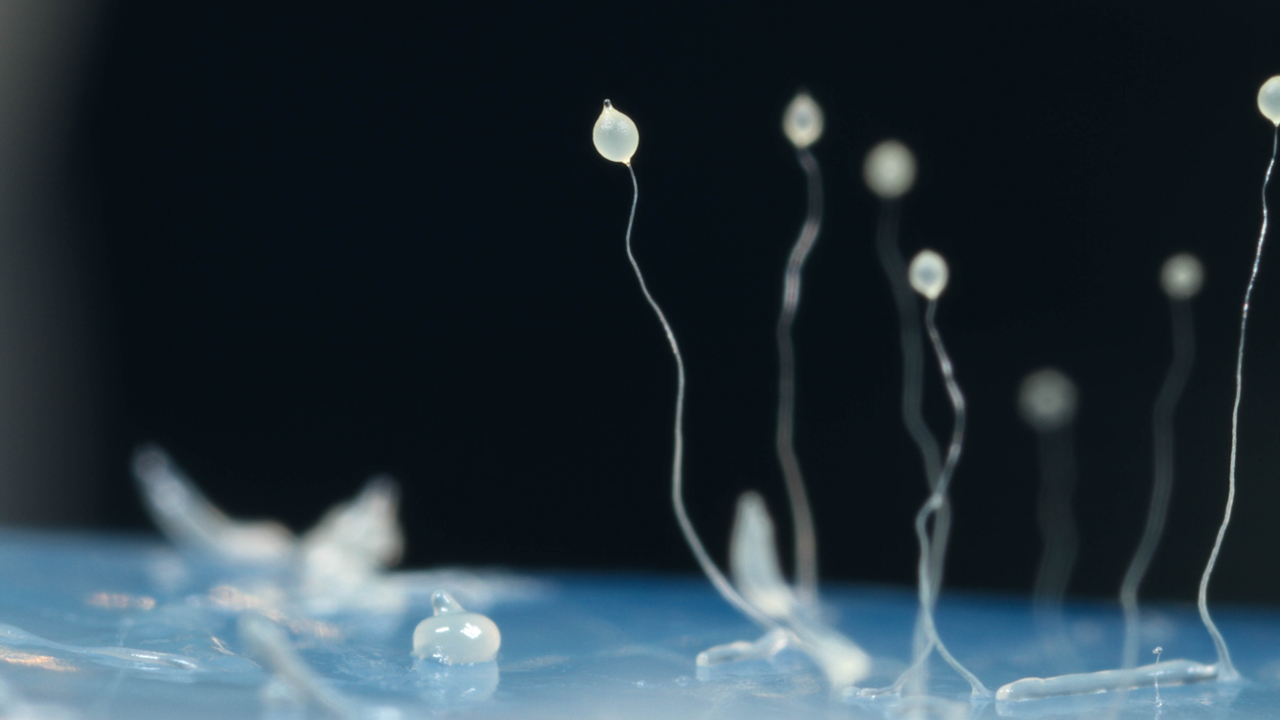
Some of Margaret Titus’s students are a little taken aback to learn how much they have in common with the slime mold Dictyostelium discoideum. Called “social amoebae” for their habit of joining together to save themselves when food is scarce, they may hold the key to understanding how a host of human cellular processes work—or go awry.
When human white blood cells or metastasizing cancer cells move through our bodies, or when nerve cells are forming connections with each other, they send out slender exploratory extensions called filopodia. And when “Dicty” cells search for food, they do the same thing. If any of these cells’ filopodia pick up signals that point them in a favorable direction, the rest of the cell follows; this is what directs their movement to the food source.
“I call filopodia ‘the cat’s whiskers of a cell,’” says Titus, a professor in the Department of Genetics, Cell Biology and Development. “Proteins called myosins, which act as mo-tors for many cells, are needed to make them, and we found the same basic operating principles in filopodia-forming myosins used by both Dicty and humans.”
This makes Dicty an excellent—and easy to use—model for learning how to stop filopodia formation in order to, for example, thwart metastasis or modulate neuronal wiring or white blood cell activity.
Best Foot Forward
The evolutionary lineages of slime molds and humans split at least 600 million years ago, says Titus. But among features that were highly conserved are myosins, some forms of which power muscle contraction in humans. Myosins play a key role in forming filopodia in both humans and Dictys.
Titus studies a Dicty myosin called DdMyo7. Early in the response to a food signal, molecules of DdMyo7 move to the area of the cell’s outer membrane facing the direction in which the cell will move. There they anchor themselves to the membrane, in the midst of a network of short filaments made of actin, another key protein.
“This actin network is like a skeleton giving shape to the cell,” Titus explains.
Next, the myosin reorganizes or reorients the actin filaments so they point in the desired direction. The myosin then works with another protein that bundles the actin filaments and prepares them to grow longer.
“This produces a bundle of about 30 filaments,” Titus explains. “As they grow, they push against the membrane, extending it into a filopodium.”
Heads and Tails
Typical of myosins, DdMyo7 has a “head” section that interacts with actin and a “tail” part that, among other things, is involved in attaching the myosin to the membrane. This ability appears crucial in allowing the cell, in turn, to adhere itself to surfaces in order to pull itself along.
“We focus on the tail section of our myosin,” says Titus.
Recently, she and her collaborator Anne Houdusse, a structural biologist at the Curie Institute in Paris, found a key similarity between the tails of the Dicty myosin and a myosin found in virtually all human cells. Then Titus and her colleagues discovered that the part of the human myosin tail that contained the similarity could be swapped into the slime mold myosin without loss of function.
This meant that the myosin tail was so important, evolution had conserved its structure for 600 million years. It also meant the slime mold myosin could model the human variety and allow research on how to help or hinder filopodia formation in disease conditions.
“This finding suggests that the tail part alone may be an Achilles heel that could be used to thwart metastatic cancer cells in humans,” Titus says.